Walter Clement Noel was famous in the wrong circles for the wrong reasons. He died in Grenada in 1916 aged just 32.
Over fifty years later, in the first decade of my life, Chitty Chitty Bang Bang was far and away my favourite film. I must have seen it six or seven times, a huge tally in the days before VCRs and DVDs. The magical tale of endangered children rescued with the help of a flying car captivated my boyish mind. I was entranced too by the outlandish inventions of the children’s father, Caractacus Potts–played by Dick van Dyke–and especially by the ingenious contraption of wheels, rails, levers and cords that he devised to cook and serve up sausages and eggs for breakfast.
I remember lying awake at night trying to figure out how to concoct a similar mechanism to hoist the books and toys from the shelves at the far side of my room without having to get out of bed.
Here comes breakfast.
But I never managed to figure out how to do it. Years passed and I grew up. When that happens, you are supposed to put away childish things. But I became a scientist.
I became a scientist because in my final year as a physics undergraduate I attended lectures in biophysics and learned about a device that is stranger and more complex than anything dreamed up by Caractacus Potts: a protein called haemoglobin.
The red blood cells that give your blood its colour do so because they are stuffed with haemoglobin, a molecule made up of four protein chains (two α and two β, shown in red and cyan in my figures)–each of which folds into a sub-unit that grips an iron-bearing haem group. The complex assembly of haemoglobin allows the four haem groups to snag four molecules of oxygen when the blood traverses the capillaries threaded through the lungs. Haemoglobin slowly releases these molecules as the blood circulates through the body, thereby providing every cell with the oxygen needed to burn the fuel that energises life.
Unfortunately for Walter Clement Noel, it was a defect in his haemoglobin molecules that probably took his life away.
To this day the story of haemoglobin is taught in biophysics courses because it was one of the very first proteins to be studied by protein crystallography, a technique in which the scattering of X-rays into myriad beams by crystals made of pure protein is interpreted mathematically to reveal the molecular architecture in exquisite detail. In the case of haemoglobin, which is constructed from over four thousand atoms (1), this architecture is astonishingly complex.
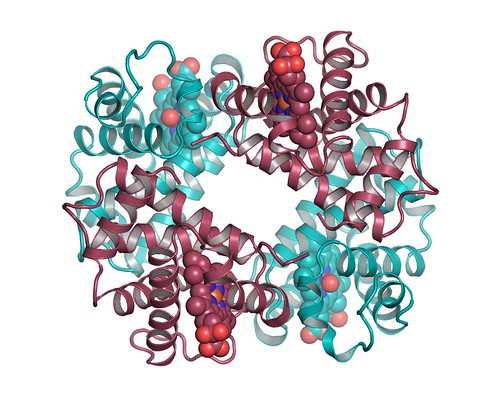
Need oxygen? You’ll want lots of these. A hugely simplified view of haemoglobin. The atoms of the haem groups are shown as spheres.
The structure of haemoglobin was published in 1959, but the effort to determine it had begun more than twenty years earlier in 1936 when Max Perutz, a diminutive Austrian Jew, paid a visit to his cousin’s husband (2).
Perutz had gone to England to escape anti-semitic persecution and to pursue his studies in organic chemistry, and found himself under the supervision of Desmond Bernal, a colourful Irish scientist who was pioneering the development of X-ray crystallography. Guided by Bernal, the young Austrian became interested in using crystallography to elucidate the molecular structures of organic chemicals. He thought could see a gap in the market–no-one had attempted to determine the structure of haem, the organic molecule at the core of haemoglobin–and, excited by this prospect, used his family connection to set up a meeting with Felix Haurowitz, a biochemist who worked on haemoglobin in Prague.
But Haurowitz was unimpressed. Haem had been synthesised some years before, so the chemical formula was known and the structure would not add much new information. Instead he encouraged Perutz to go after the structure of the whole haemoglobin molecule. At the time, this was a monumental proposal–the techniques did not exist to tackle a structure containing a hundred atoms, never mind one composed of several thousand.
That didn’t seem to matter–Perutz was inspired. And fortunately perhaps, he was also young enough and naive enough to think the project feasible. He knew Bernal was keen to work on proteins and had recently developed methods for recording high quality X-ray diffraction patterns from protein crystals, which are much more delicate than the crystals of organic molecules that chemists were used to handling. Haurowitz could also offer immediate encouragement since he himself had grown crystals of haemoglobin, taking his cue from the work of two American chemists, Edward Reichert and Amos Brown.
Haemoglobin had first been crystallised in the nineteenth century but Reichert and Brown, working at a near-industrial scale, published a monograph in 1909 containing over 600 photographs of crystals grown from haemoglobin purified from 109 different species of animal. Though it was too early to know anything of the structure of the protein molecules, since their work predated the X-ray diffraction analysis of crystalline matter, Reichert and Brown noted that variations in the shapes of the crystals of the haemoglobins could be correlated with the apparent relatedness of different animal species. Presciently, they reasoned that the variations in crystal shape probably reflected small differences in the haemoglobin molecules themselves and claimed it as molecular evidence–the first–for Darwin’s theory of evolution. But Reichert and Brown had nowhere to go with their observation since their work preceded by more than thirty years Oswald Avery’s determination that DNA–and not protein–is the material basis of genes and heredity.
At around the same time the Chicago physician James Herrick saw with his microscope the unusual distorted shapes of Walter Clement Noel’s red blood cells that were the cause of his painful anaemia. But he was unable to make any connection with structure of the resident haemoglobin. Like Reichert and Brown, in the absence of structural information, he had nowhere to go.
Back in Cambridge Perutz was not stuck. He soon learned how to grow crystals of haemoglobin but it would take more than twenty years of hard labour–interrupted by the war that saw him first interned as an enemy alien and then diverted to work on Habbakuk, a doomed scheme to build an aircraft carrier out of reinforced ice–before the structure of the protein finally emerged. In large part the difficulty was the one that was obvious from the beginning–the need to develop the methodology for extracting the structure of such a large molecule from crystalline X-ray diffraction patterns. In this endeavour Perutz experienced a moment of glittering genius: he predicted that the addition of heavy metal atoms–in his case, mercury–would produce small but measurable changes in the diffraction that could be used to solve the problem. But when the day came to test his idea there was no sense of a cold, scientific mind at work. Perutz suffered every particle of the excitement and fear that any of us senses when our hopes are pinned on the outcome of a crucial experiment:
”As I developed my first X-ray photograph of mercury haemoglobin, my mood altered between sanguine hopes of immediate success and desperate forebodings of all possible causes of failure. I was jubilant when the diffraction spots appears in exactly the same positions as in the mercury-free protein, but with slightly altered intensity, exactly as I had hoped.”
The structure that was finally published in 1959 was rather crude. It revealed just the outline form of the molecule and was at first something of a disappointment to Perutz. But over the next few years the full atomic structure of haemoglobin emerged from the murk of ignorance and was revealed to be a thing of glorious intricacy.
One could finally see exactly how the atoms of the protein chains curled into helices that wrapped around one another to give haemoglobin a shape that clasped onto each of the four haem groups. But more than that, comparison of structures solved with and without oxygen bound to the protein showed that haemoglobin is a moving molecular machine of great subtlety, one that is finely tuned to its oxygen-transport function.
The looping video above gives an idea of the contortions that haemoglobin undergoes as it binds and releases its life-giving cargo. Below you can have a closer view of the writhing and shifting of the atoms involved (3).
In a lecture theatre back in 1982 the realisation of the complexity contained within a single protein molecule produced a writhe and a shift in my mind and set me on the course that–albeit circuitously–made me a protein crystallographer. It has been boggling my mind ever since. I am still tickled by the intricacy of the mechanism, which is why I so much wanted to share it. I toiled longer than I care to admit to generate these morphing videos and, though I very much hope they show you something of the ‘life’ within just one protein, the motions shown are just an approximation (3) and the mechanical repetition of the looped animation hides the truer, stochastic nature of the molecular contortions. The scientist in me fears that I may be mis-leading you, but I’m taking that risk.
I’m not going to lay out the full explanation of how haemoglobin breathes and flexes as oxygen attaches to the centre of each haem group; this blogpost is already too much like a textbook. But as the oxygen binds to the iron atom in the centre of the haem group, that atom shifts and the whole plane of the haem flexes; the side-chain of a histidine amino acid in the protein that is bonded to the iron atom is pulled toward the haem, a movement that levers the helix containing the histidine to a new position.
In this way, because the helix extends to the interface with the adjacent sub-unit, there is communication from one part of the molecule to another. In this way haemoglobin senses when oxygen levels are high and responds by adjusting the conformation of its sub-units to increase its binding affinity.
Thus can haemoglobin morph into a high-affinity form as the blood cells pass through the oxygen-rich lungs and grab tightly onto four molecules of oxygen, its full complement. As the red cells travel to the extremities of the body the oxygen is slowly released. This in turn relaxes haemoglobin back to the low-affinity form, which enhances the discharge of oxygen, maximising the efficiency of transport.
But oxygen isn’t the only molecule to exert control over haemoglobin. The protein is also sensitive to the local carbon dioxide concentration, which is elevated in very active tissues. Carbon dioxide binds to and modifies the protein to further reduce its affinity for oxygen and induce additional release in those parts of the body where it is most needed. This is the Bohr effect, named for its discoverer Christian Bohr, the father of the more famous Niels, who laid the foundations of atomic theory and quantum mechanics.
And the layers of complexity don’t end there. Diphosphoglycerate (DPG) provides yet another means for our bodies to regulate oxygen binding to haemoglobin. The analysis of the structure shows that DPG binds to the crevice between the two β sub-units of the protein and drives it towards the low affinity state. Paradoxically, by reducing the affinity of haemoglobin for oxygen, DPG allows us to survive better a low oxygen levels, again by enhancing the release of bound oxygen in the deep recesses of the body. Mountaineers pausing to acclimatise to high altitudes are giving their bodies the time boost levels of DPG so that they can function in the thin atmosphere.
The sheer cleverness of the complex mechanism for transporting life-giving oxygen revealed by Perutz’s structural work makes me smile every time I think of it. But lest you should think that I am about to shade into acquiescence to any kind of intelligent design, let me tell you about a darker side to haemoglobin.
For the structure of the protein also explains exactly how a mutation in Walter Clement Noel’s gene for the haemoglobin β-chain that changed just one amino acid–at position 6 in the chain–was sufficient to cause the painful anaemia that plagued his life and very probably led to his early death.
That mutation replaces the water-loving OH side-chain of a Serine amino acid with a somewhat stickier Valine side-chain that prefers to be shielded from water. The structure shows that the Valine side-chain can fit snugly into a sticky pocket on the surface of the β-chain on another haemoglobin molecule that becomes exposed in its deoxygenated form. But because each haemoglobin molecule has two β-chains, this interaction can daisy-chain, leading to the formation of long fibrils of haemoglobin, especially deep within the body where the molecules are more likely to have dumped their oxygen cargo. These fibrils bundle into fibres long enough and strong enough to distend the red blood cells into the unusual crescent or sickle shape that Herrick could see in his microscope.
(The figure at left shows a small section from a haemoglobin fibril. The atoms of the valine side-chains that stabilise the fibril like the teeth of a zip are shown as orange spheres).
Healthy red blood cells have a dimpled discoid shape and slip easily, often in single-file, through the finest capillaries of the body. But sickled cells get stuck, causing painful blockages–especially in active tissues that are depleted of oxygen. The distorted cells are usually eliminated by the spleen, leading to the anaemia that Noel suffered from.
This sickle cell anaemia is most common in people–like the West Indian Noel–who originate from central Africa, where it was better known by different appellations. The incidence is surprisingly high (about 0.2% among African Americans, but probably higher in Africa) for an inherited disease that leads to an early death. Why should a mutation that causes such a debilitating disease persist in the human population?
Bluntly, brutally, Walter Clement Noel suffered debilitating anaemia because it was a price he had to pay so that others could be resistant to the scourge of malaria. Noel was unlucky because both copies of his haemoglobin β-chains carried the sickling mutation, making him particularly vulnerable to the distortion of his red blood cells and the painful symptoms that ensued. In his case unfortunately, the ‘cure’ was worse than risking malarial infection from mosquito bites.
But in individuals with a single copy of the defective haemoglobin chain, the sickling occurs only very rarely–they live essentially disease-free (4)–but it is induced if their red blood cells become infected with the malarial parasite. The infected cells therefore tend to sickle and this aids elimination of the infection, although the mechanism is not properly understood: the parasite may be eliminated along with the distended red cells in the spleen or the haemoglobin fibres may simply make the cell too leaky for it to replicate. Whatever the precise mechanism, the mutation provides an important advantage in populations exposed to the threat of malaria.
But not everyone is protected. The demise of Walter Clement Noel from complications arising from sickle cell anaemia tells us is that evolution is a pitiless force of nature; it looks only to the good of populations and cares nothing for individuals.
Noel’s tragedy is all the more acute because he probably had a perfectly serviceable replacement for the defective β-globin chains within him: the γ-globin chains that he had used as a foetus growing within his mother’s uterus. The foetus doesn’t breathe air but instead must extract oxygen from the maternal blood flowing through the placenta. To do this, the β-globin gene is inactive at the foetal stage and haemoglobin is instead formed by pairing two α and two γ-chains, which gives it the increased affinity needed to compete for oxygen with its mother’s blood. But once the child is born, expression of the γ-chain is turned off just as the gene for the β-chain is activated. A solution for Walter Clement Noel would have been to reverse this transition, but there was no way to do that back in 1910 (5).
The problem of the genetic control of haemoglobin α, β and γ-chains was discussed at the First Global Congress on Sickle Cell Disease, held this year in Ghana to mark the centenary of the publication of Herrick’s famous paper. New insights into the factors that regulate these genes promise to open fresh avenues in the search for therapeutics. But progress is slow–connections between observations are not so readily made–and fully effective treatments have yet to be developed. It was my reading the report of the meeting a couple of weeks ago in Science that triggered the recollections that loop through this blogpost. Unfortunately the report also makes clear that after one hundred years of work on this–the first molecular disease–people are still suffering and dying.
Footnotes
(1) This tally does not include hydrogen atoms.
(2) See Max Perutz and the Secret of Life by Georgina Ferry. 2007. Chatto & Windus.
(3) The animations are morphs between the two known structures of haemoglobin with and without oxygen bound (PDB codes 2dn1 and 2dn2 respectively) and were made with eMovie. It is not a physically exact representation of how the conformational change is achieved but it is probably a reasonable estimate. Sharp-eyed readers will see that I have omitted the iron atom in the centre of each haem group. This was necessary because the morphing program–for reasons that I couldn’t fathom–failed to deal with them in a reasonable way.
(4) However, people with a single faulty β-chain are advised not to climb to high altitudes or to otherwise expose themselves to low oxygen levels since this promotes the aggregation of their haemoglobin.
(5) Since 1995 hydroxyurea has been used to offer relief from painful sickle cell crises in some individuals; it appear to work in part by re-activating the foetal γ-chain gene.








Brilliant post Stephen. Open Lab nomination.
“Years passed and I grew up. When that happens, you are supposed to put away childish things. But I became a scientist.”
and I love this 🙂
Damn, Ian, you took the words right out of my mouth. Or my fingers. Whatever.
Stephen – great structure – suspense, even! Really well done, among your many so-excellent posts. And you still have time to go refute woo peddlers. Do you leap tall buildings in a single bound, too?
Thanks Ian. I’ve been fiddling with this one for a couple of days and finally decided to release it into the wild. It’s somewhat, ahem, longer than my usual fare but I hope that won’t put off too many readers.
Slightly annoyingly I saved a draft version earlier today (about 2 am) but when I published this evening that time stamp seems to have stuck. So it first appeared half-way down the list of Recent Posts on the front page. Another MT4 bug to log, I guess.
@Heather – 🙂
Sorry to burst that bubble but I can only cope with tall buildings if I’m am safely inside them. Heights and I are not on friendly terms.
Meh! beta-chain schmeta-chain! Throw away all your chains! Abnormally low levels of oxygen, you say? Dontcha know that blood, which is mostly water, must retain the memory of the oxygen that was once dissolved in it? As and when the external O2 tension goes down, the oxygen-memory of blood-water becomes stronger and stronger. If you don’t trust me, ask the Nobel Laureate and parapsychology researcher from Cambridge U, Brian D Josephson. Booiyaah!!
[/I feel dirty now, must go and wash my mouth/fingertips with some 2N HCl…]
@Dr. Curry, that was an awesomely brilliant write up – I completely echo Heather’s admiration.
A barnstorming post, Stephen. I am in awe.
I keep meaning to write about Perutz’s Habakuk project. Much of the research took place beneath Smithfield meat Market in London. It’s a fascinating tale.
And the MT bug isn’t a bug – it’s just how MT is, on any system. The timer defaults to when you first started the post unless you tell it otherwise. Unless you did, in which case it is a bug.
Fantastic post Stephen; haemoglobin was always one of my favourites.
@Kausik – 🙂 Time for us all to calm down on the homeopathic front. And thanks.
Cheers Matt – very kind. Not a bug eh? Well it seems like an odd default choice to make the date/time of posting the point at which the draft was first saved. I guess you had warned me way back when that MT4 was far from an ideal solution to all our bloggery problems. At least now I’m aware of that particular issue.
And thank you, Jim. I imagine we’re not the only ones to have fallen for Hb.
Brilliant post. And as somebody treating patients with blood disorders, including the occasional patient with sickle cell anemia, I can particularly relate to this story.
There are still so many things to learn about hemoglobin. One of only six abstracts selected for the plenary session of the 2009 American Society of Hematology Meeting was about the “control of hemoglobin gene switching via BCL11A”:http://ash.confex.com/ash/2009/webprogram/Paper21895.html.
Attending the Lindau Nobel Laureate meeting last month made me appreciate even more the historical perspective of science. There are so many fantastic stories to tell.
Great stuff Stephen! The sickle cell anemia / malaria story fascinated me in high school – I guess the story that set me on the path to studying evolutionary genetics was just a different part of the same story that made you a protein crystallographer!
BTW, as excellent as this post is (and it really is), I’m most intrigued by the “doomed scheme to build an aircraft carrier out of reinforced ice”! M@, please do write that post some time!
Cheers Martin – always good for us bench jocks to have the clinician’s perspective.
The role of BCL11A was discussed in the report of the Accra meeting that I linked to at the end of the post. It appears to silence the &gamma-globin genes and switch on β-globin expression after birth. If there was way to silence BCL11A itself in the body, either through RNAi or the use of some kind of protein inhibitor, that could be an effective gene therapy for sickle cell disease. It’s still a promising theory rather than a solution, but at least it gives us a clear target to aim at.
Cath – in that case: snap! 😉
I second your request of Matt to write up an account of the project (though the Wikipedia pape is a good place to start). I first came across it in the biography of Bernal who, though less well remembered as a crystallographer than Perutz, was by all accounts a much more charismatic figure. He was also involved in Habbakuk and several other grand schemes during WWII (including the Normandy landings).
Dr. Curry, I accept your rebuke. I am trying to let it go… But that thread must have left me scarred for life!!
Please Kausik, call me Stephen…! I like to keep things informal around here. 😉
I never saw the Chitty-Chitty-Bang-Bang film, but read the book in grade 2 or 3 (as soon as my English was good enough and after seeing the rest of the students read it) and vaguely remember the inventions! This was around the time I wanted to be “an inventor” when I “grew up” so now I’m starting to think the two may have been related in some way or another.
@Eva – Your comment reminded me of the rather surprising that the book was written by, Ian Fleming, the creator of James Bond.
I agree with your other point: I think that the sorts of people who are taken with gadgets and machinery as children are perhaps of that cast of mind that makes for a half-decent scientist.
Hi Stephen,
I just wanted to let you know that I have included this post in the latest Scientia Pro Publica carnival over on my blog. Do drop by when you have a moment.
cheers,
Madhu
My goodness, how did I miss this the first time around? Fantastic. Glad you pointed us this way from your recent post.
Cheers Richard.